Machine Learning in Healthcare
The 16th CAIML online meeting was held on January 19. We had a presentation by Annika Pick on applying ML to challenging clinical data, followed by a Q&A session and the opportunity to get to know each other and share ideas in a networking session.
Speaker
Annika Pick, Data Scientist at Fraunhofer IAIS
A great #CAIML session with Annika Pick on applying #ML to challenging #clinical data. Annika's work at #Fraunhofer #IAIS: "Aligning Subjective Ratings in Clinical #DecisionMaking": https://t.co/eZ1LU24s2S
See you in March @CologneAIML no. 17#AI #KI #MachineLearning #Meetup pic.twitter.com/gd3xI9T8Lq
— Thomas Fabula (@TFConsult) January 20, 2021
Topic
Applying ML to challenging clinical data – how to align subjective disease activity with objective indicators.
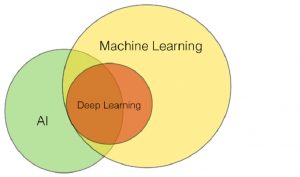
CC BY-SA 4.0: Fig-y Part of ML as subfield of AI or AI as subfield of ML (Wikipedia)
Healthcare Analytics
The Healthcare Analytics team at Fraunhofer IAIS develops applications based on Data Analytics and Machine Learning to discover patterns in clinical, pharmacological and other types of medical data.
The analysis of such data is in particular challenging due to small sample sizes, large numbers of variables (high dimensionality), frequently missing values and abundant noise.
Scope
“Recently, we have adapted the Ranking SVM method in order to align hundreds of objective symptom assessments with the subjective rating of disease activity performed by a trained physician.
We applied this approach in the context of the disease Psoriatic Arthritis and presented the results at the ECML-PKDD PharML workshop (https://arxiv.org/abs/2009.06403).
In this meetup, I will introduce the use case, provide a short overview of the (Ranking) SVM method and show you how we applied it to create a comprehensive score that incorporates both the physicians’ domain expertise and symptom indicator variables.” ~ Annika Pick
Agenda
- Start of the meetup via MS-Teams and short introduction
- Annika’s talk on Applying ML to Challenging Clinical Data
- Collect questions via sli.do
- 15 minutes Q&A and Networking Session via “wonder.me”
CAIML – Cologne AI and Machine Learning
www.meetup.com/de-DE/Cologne-AI-and-Machine-Learning-Meetup
PharML 2020
Machine Learning Applications in Pharma and Healthcare a workshop taking place at ECML PKDD 2020, September in Ghent, Belgium.
“Progress in machine learning and artificial intelligence will be critical in the coming years to better understand the mechanisms of disease, which will in turn enable us to create more efficacious therapies for patients. The drug development cycle entails many steps where large amounts of valuable data are collected in the context of clinical trials. Working on this data provides us with potential treatment targets, new biomarkers, as well as other information that enables us to identify which patients will benefit most from a given treatment. Additionally, safety and efficacy information is collected. After a drug enters the market, further data is generated and collected in the form of electronic medical records, disease registries, health insurance claims, surveys, digital devices and sensors, among others …” ~ PharML 2020
https://sites.google.com/view/pharml2020/home
Paper
Annika Pick (Fraunhofer), Sebastian Ginzel (Fraunhofer), Stefan Rüping (Fraunhofer), Jil Sander (Fraunhofer), Ann Christina Foldenauer (Fraunhofer) and Michaela Köhm (Fraunhofer). Aligning Subjective Ratings in Clinical Decision Making